Who We Are
Founded in Finland in 2000, Nexstim is a growth-oriented medical technology company operating in the international market. Nexstim has developed a world-leading non-invasive brain stimulation technology for navigated transcranial magnetic stimulation (nTMS) with highly sophisticated 3D navigation providing accurate and personalized targeting of the TMS to the specific area of the brain.
Nexstim has subsidiaries in the United States (Nexstim, Inc.) and in Germany (Nexstim Germany GmbH).
Over 250 NBS systems and over 110 systems that include therapy functionalities have been delivered to facilities worldwide for neurosurgical planning, multiple therapies, and research.
What We Do
Diagnostics
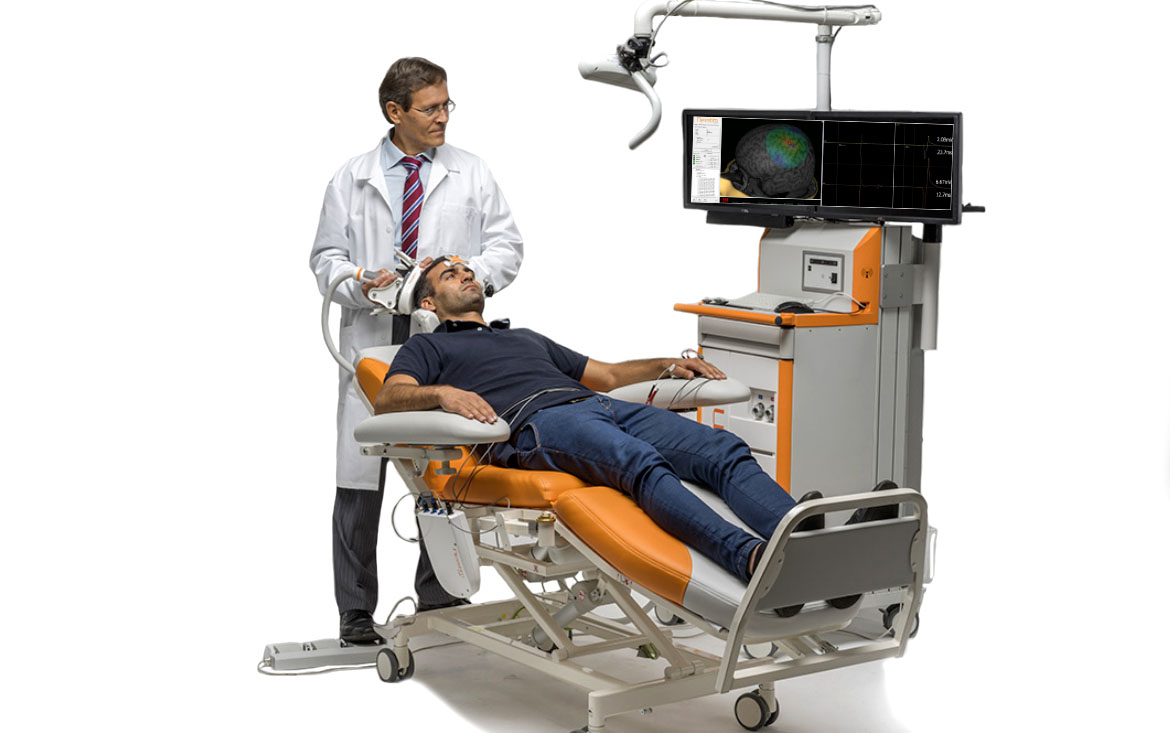
One of the most crucial pieces of information needed for neurosurgery is the tumor’s or other brain lesion’s location in relation to the essential functions and their connections in the patient’s brain. nTMS mapping with Nexstim’s system is used when the tumor is thought to be close to important motor and language areas in the patient’s brain. These brain maps are useful when deciding the treatment option. Clinical data has been generated demonstrating the value of Nexstim’s unique navigation system for pre-surgical mapping with regard to patient outcomes.
Key authority approvals (NBS System 5):
- FDA-cleared for presurgical mapping of the speech and motor cortices of the brain.
- CE-marked for presurgical mapping of the speech and motor cortices of the brain.
Therapy
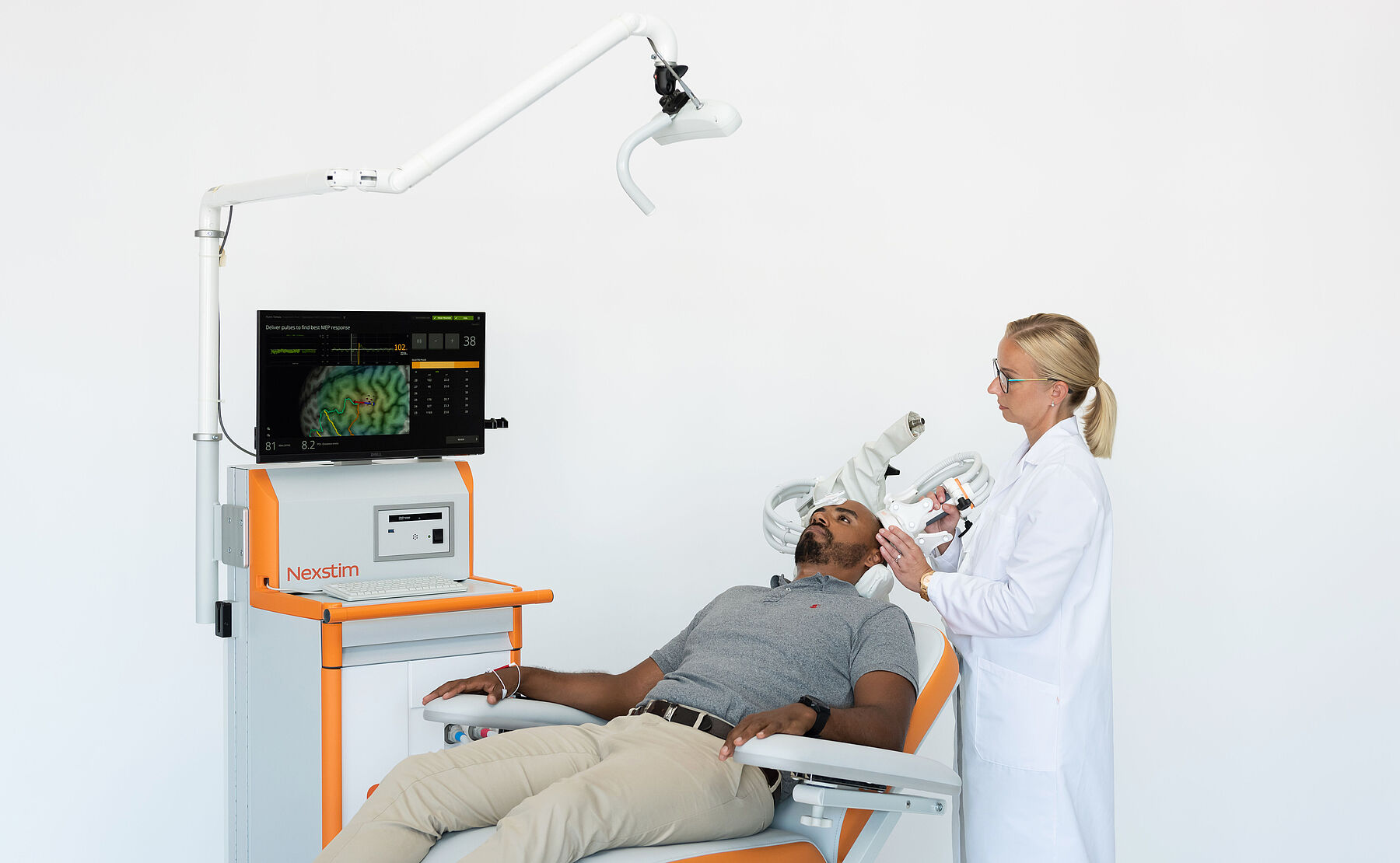
If pharmaceuticals are not working or a non-drug option is wanted, nTMS conducted with Nexstim’s system might be the answer for treatment of major depressive disorder or for chronic neuropathic pain. Nexstim launched its Navigated Brain Therapy (NBT®) system in 2018 in the United States for the treatment of MDD, following FDA clearance in 2017. Launched for therapy use in 2023, the NBS 6 is a new generation Nexstim system with a modular system concept that makes it easy to add new features to existing systems.
Key authority approvals (NBT® System 2 and NBS System 6):
- FDA-cleared for the treatment of major depressive disorder
- CE-marked for the treatment of major depressive disorder and chronic neuropathic pain.
Customers have the possibility of acquiring a system that has either diagnostic or therapy functionalities, or a system combination that enables the delivery of both applications in the same system.
Research and Neuroscience
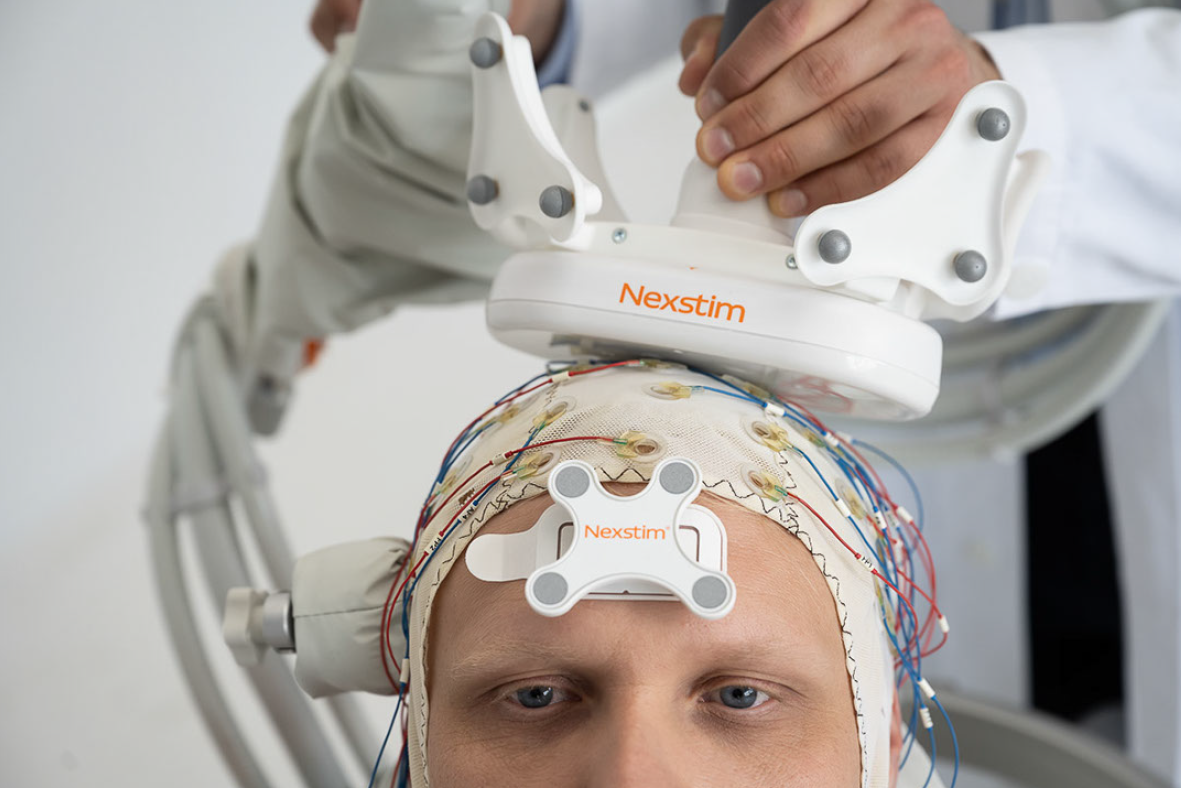
- The core of the Research and Neuroscience business is the sale of Nexstim systems and support and development services to leading research hospitals and clinics, in order to support leading expert customers (key opinion leaders, KOLs) working in these institutions at the forefront of technological and clinical development.
- The international research community is particularly interested in developing therapies based on the stimulation of connections/neural networks between different areas of the brain. Nexstim's unique electric field neuronavigation has proven to be particularly useful and necessary in this regard.
- TMS-EEG diagnostics and treatment development based on this methodology are a growing area of interest in research. Here, too, neuronavigation and Nexstim's real-time EEG (electroencephalography) software are particularly useful and are attracting growing interest.
Summary of CEO Mikko Karvinen's review of H1 2025:
This is a summary of the CEO's review. To read it in full, please visit the financial reports page.
"Nexstim continued its positive trend in terms of net sales growth and improved results during the first half of 2025. In H1 2025, we achieved total net sales of EUR 4.5 (3.2) million, representing growth of 41.8%.
The Diagnostics business grew by 65.3% during the first half of 2025, with net sales of EUR 2.5 (1.5) million. We delivered a total of seven new diagnostic systems during H1 2025.
Net sales from the therapy business decreased by 8.4% during the first half of 2025 and amounted to EUR 1.4 (1.6) million. The decline was mainly due to a decrease in system sales in the Therapy business, which fell by 37.0% compared to the same period last year. During H1 2025, we delivered one purely therapeutic system to Europe and the rest of the world. This development was mainly since most of the therapy applications were delivered in connection with Diagnostics and Research and Neuroscience system.
With the new strategy period, we now also report separately the net sales of the Research and Neuroscience business, which grew by as much as 565.7% during the first half of 2025, amounting to EUR 0.6 (0.1) million. This rapid growth was due to the sale of two research systems and a EUR 0.2 million license fee from Sinaptica's exclusive agreement.
In addition to the above-mentioned system already delivered, we had an open order backlog of eight systems at the end of June 2025.
Strategic focus remains on growth and profitability
In line with our strategy for 2025–2028, Nexstim will continue to enable individualized and effective treatment and diagnostics for patients with severe brain diseases and disorders.
In line with the main objective of Nexstim's 2025–2028 strategy, we have focused on profitable net sales growth, and our sales forecast for full year 2025 looks promising. This growth is now supported by our global distributor partner network, which was strengthened at the end of last year by the Brainlab cooperation agreement. We are now looking to take immediate advantage of this, particularly in the marketing and sales of diagnostics systems in the United States and Europe. In early 2025, we developed and trained our partner network for the Diagnostics business in close cooperation with Brainlab. This will enable us to effectively and comprehensively deliver our sales and marketing message to as many markets as possible among Brainlab's thousands of existing customers. In addition to this collaboration, Nexstim's direct sales and marketing still have significant opportunities for developing the Therapy business, research and neuroscience customers, and the clinic partner network.
Our second strategic objective for 2025 is to successfully support the long-term collaboration between Nexstim and Brainlab and to expand our network of other partners and clinics, primarily in the United States. When it comes to the Brainlab collaboration, the parties have also agreed on certain gross margin targets for payments that Nexstim will receive from the sale of its products (including maintenance agreements) in Brainlab's business area. The gross margin target set for 2025 is EUR 4 million. The EUR 2 million portion of the gross margin target allocated to the first half of 2025 was not yet fully achieved, and a gross profit insurance payment of EUR 0.1 million was recognized as part of the Diagnostics business's net sales for the period.
With the expansion of the network of partner clinics, Nexstim's system will be installed in neuroscience clinics, and new investments may be made in clinic service companies in the large US market. Nexstim Investments, LLC, Nexstim's venture capital company, aims to expand Nexstim's network of partner clinics in the United States in collaboration with carefully selected leading experts in the field. We will continue to develop our cooperation model with experts, enabling the treatment of more patients in the United States with Nexstim's TMS technology. During the strategy period, we aim to increase the number of Nexstim systems and treatment sessions at our partner clinics.
Our third strategic objective for 2025 is to launch the new NBS 6 diagnostic system in our main markets, enabling ease of use and future add-on modules in the same system. The development and launch of a new product generation will continue to be an essential part of Nexstim's operations during the 2025–2028 strategy period. We will now continue to finalize the development of the system and progress regulatory approvals during 2025 so that, in line with our strategic objective, we will also have diagnostic applications launched in the key markets as part of the new NBS 6 system.
In January 2025, the company announced the start of joint development of the SinaptiStim® precision neuromodulation system for the treatment of Alzheimer's disease together with Sinaptica Therapeutics, Inc. ("Sinaptica"). The company announced that the planned delivery of the research system for the first validation testing, and future clinical trials was in March. Nexstim announced that it is developing the system according to Sinaptica's specifications, combining it with a high-resolution 64-channel electroencephalography (EEG) system from Bittium, which enables precision calibration of the therapy for each Alzheimer's patient. The new research system will be used in Sinaptica's upcoming clinical trials related to the treatment of Alzheimer's disease, with several studies planned to begin in 2025.
At the end of June 2025, the Company announced that it had signed an exclusivity agreement with Sinaptica Therapeutics, Inc. regarding the companies' planned collaboration in the field of Alzheimer's disease treatment. The signed agreement covers plans for 2025. The final agreement is expected to be drafted and signed before the end of the year. In June 2024, the Company announced that it had signed a letter of intent with Sinaptica regarding the development, manufacture, and supply of Sinaptica's patented precision neuromodulation system for the treatment of Alzheimer's disease and MCI (mild cognitive impairment). The system is based on Nexstim's NBS 6 medical TMS and neuronavigation systems and related software, including integrated EEG software. As part of the signed exclusive agreement, Sinaptica undertakes to order the research system it needs for clinical trials from Nexstim, and Nexstim will not commercialize its technology in the field of Alzheimer's disease independently or with partners other than Sinaptica. The agreed exclusivity is conditional upon payments of EUR 1.5 million in multiple installments to Nexstim during 2025. The planned partnership would be a global, 10-year exclusive arrangement. The financial structure of the partnership consists of a signing fee for the disclosed exclusivity rights, a milestone-based development project, and the sale of clinical and commercial system. The milestone-based project and purchases of clinical system are defined in more detail in the final agreements, which may be subject to change during negotiations. The long-term exclusive rights arrangement also depends on payments to be made by Sinaptica during 2025 and the signing of final agreements before the end of 2025.
Building shareholder value while taking exceptional circumstances into account
Despite the tense global political situation, our expectations for the rest of 2025 are positive in many ways. Our current business is heavily focused on the EU and the US, but with our new partners, we will continue to actively expand Nexstim's technology into the Asian market. Nexstim will continue its determined efforts to develop personalized and effective methods for the treatment and diagnosis of severe brain diseases and disorders. We strongly believe that our work will support long-term shareholder value growth in the form of increasingly strong competitive advantages, rapid growth, and improved financial results."
Mikko Karvinen, CEO
Page last updated: 19 August 2025



