nTMS motor mapping in pediatrics
- The youngest reported patient successfully mapped was 8 weeks of age1
- Conducted in a child-friendly environment without the need for sedation
- May reduce the need for two-staged cases for invasive functional brain mapping1
nTMS language mapping in pediatrics
- The youngest reported patient successfully mapped was 4 years of age1
- Bilateral mapping captures inter-hemispheric functional reorganization1
- Multilingual mapping enables visualization of cortical regions associated with each language5
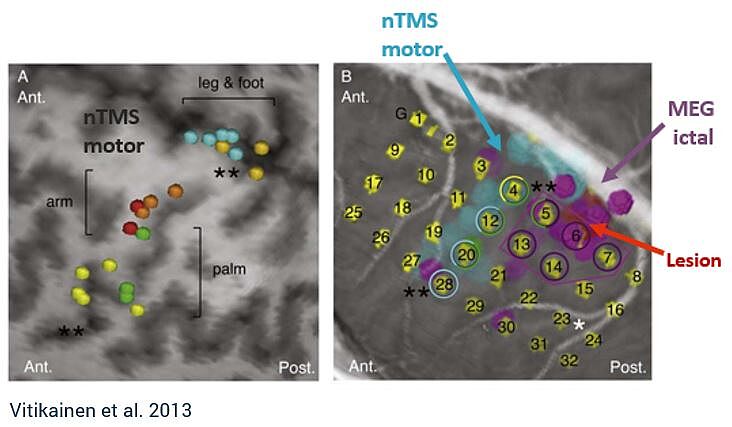
nTMS & MEG: A powerful combination in epilepsy surgery
Combining MEG and nTMS can yield powerful results. MEG can be used to investigate the onset zone for epileptic seizures, whereas nTMS can provide the margin to functional tissue, providing safe resection zones.4 This combination may reduce the number of patients required to undergo two-staged cases for invasive ECS monitoring, which carries significant health risks.4,6
“[MEG and nTMS] can be added to the standard preoperative work-up and may even hold a potential to replace the ECS in a subgroup of patients with epilepsy who have the suspected epileptogenic zone near the sensorimotor cortex and seizures frequent enough for ictal MEG.” - Vitikainen et al.
How nTMS is used in practice
See how one major epilepsy program in the US is using Nexstim nTMS motor and language mapping preoperatively to advance epilepsy care. This article also highlights how nTMS provides unique advantages to the pediatric population.
For children with epilepsy or brain tumors, nTMS can facilitate timely surgery which could otherwise be delayed, because other mapping methods were infeasible or unsuccessful.
Shalini Narayana, MD, Professor of Neurology,
Le Bonheur Children's Hospital, United States.
Learn more about how nTMS is used by our community of experts:
Yes, I would like to know more
References
1. Narayana, S. et al. Clinical utility of transcranial magnetic stimulation in the presurgical evaluation of motor, speech and language functions in young children with refractory epilepsy or brain tumor: preliminary evidence. Front. Neurol. 12, 664782 (2021).
2. Raffa, G. et al. The role of navigated transcranial magnetic stimulation for surgery of motor‑eloquent brain tumors: a systematic review and meta‑analysis. Clin. Neurol. Neurosurg. 180, 47–53 (2019).
3. Pasichnik, A. et al. Discrepant expressive language lateralization in children and adolescents with epilepsy. Ann. Clin. Transl. Neurol. 9, 597–607 (2022).
4. Vitikainen, A.-M. et al. Combined use of non-invasive techniques for improved functional localization for a selected group of epilepsy surgery candidates. NeuroImage 45, 342–348 (2009).
5. Gibbs, J. W. et al. Presurgical language mapping in bilingual children using transcranial magnetic stimulation: illustrative case. J. Neurosurg. Case Lessons 2, CASE21152 (2021).
6. Önal, M. Z.et al. Complications of invasive subdural grid monitoring in children with epilepsy. J. Neurosurg. 98, 1017–1026 (2003).
Intended purpose & Indications for use
Pre-procedural mapping (CE mark, FDA clearance, for information on other regional clearances contact Nexstim):
Intended purpose: NBS 6 is intended to be used for localization and assessment of the motor cortex and motor tract integrity for pre-procedural planning purposes. NBS 6 is intended to be used for localization and assessment of cortical areas of speech function for pre-procedural planning purposes.
Indications for use: NBS 6 is indicated for noninvasive mapping of the primary motor cortex of the brain to its cortical gyrus. NBS 6 provides information that may be used in the assessment of the primary motor cortex for pre-procedural planning. NBS 6 is indicated for noninvasive localizations of cortical areas that do not contain essential speech function. NBS 6 provides information that may be used in pre-surgical planning in patients undergoing brain surgery. Intraoperatively, the localization information provided by NBS 6 is intended to be verified by direct cortical stimulation. NBS 6 is not intended to be used during a surgical procedure. NBS 6 is intended to be used by trained clinical professionals.
Post-operative Rehabilitation (CE mark, for information on other regional clearances contact Nexstim):
Intended purpose: NBS 6 is intended to be used for the treatment of surgically induced new or worsening post-operative motor deficits of the upper limb as an adjunct therapy for motor rehabilitation in adult patients having undergone brain tumor surgery. NBS 6 is intended to be used by trained clinical professionals.
Nexstim NBS 6 is not cleared by the FDA for commercial use of post-operative rehabilitation in the United States, for investigational use only.
Major Depressive Disorder (CE mark, FDA clearance, for information on other regional clearances contact Nexstim):
Intended purpose: NBS 6 is intended to be used for treatment of major depressive disorder (MDD) by targeting and delivering noninvasive repetitive TMS stimulation to the patient's dorsolateral prefrontal cortex.
Indications for use: NBS 6 is indicated for the treatment of Major Depressive Disorder in adult patients who have failed to achieve satisfactory improvement from prior antidepressant medication in the current episode. NBS 6 is intended to be use by trained clinical professionals.
Chronic Neuropathic Pain (CE mark, for information on other regional clearances contact Nexstim):
Intended purpose: In adult patients suffering from chronic unilateral neuropathic pain, NBS 6 is intended to provide electric field navigated noninvasive repetitive TMS stimulation as therapy to alleviate pain. NBS 6 is intended to be used by trained clinical professionals.
Indications for use: NBS 6 is indicated for MRI-guided and electric field (or E-field) navigated, noninvasive repetitive TMS stimulation (rTMS) of the motor cortex as therapy to alleviate chronic unilateral neuropathic pain in adult patients. Nexstim NBS 6 is intended to be used by trained clinical professionals.
Nexstim NBS 6 is not cleared by the FDA for commercial use of the treatment of chronic pain in the United States, for investigational use only.

