What is SmartFocus® nTMS?
Nexstim NBS 6 with SmartFocus® nTMS technology uses sophisticated navigation tools to visualize the location, orientation and magnitude of the maximum stimulating electric field (E-field) induced when the TMS coil is activated. SmartFocus® nTMS takes into account the unique shape and conductivity of each patient’s brain and the positioning of the coil and uses this information to determine the location and orientation of the maximum induced E-field in the brain.
In addition, advanced algorithms enable the stimulation dose to be quickly and accurately determined for each patient using their own neurophysiological readings. This all makes Nexstim nTMS a truly personalized and accurate nTMS therapy.
SmartFocus® nTMS treatment offers the patient:
- A measured and individualized dose of TMS
- TMS with the accuracy demanded by brain surgeons
- Treatment sessions personalized for the patient and his or her brain
- Assurance the patient receives his or her prescribed dose every time
- Unsurpassed safety and comfort
Why Nexstim E-Field Navigated TMS is Different?
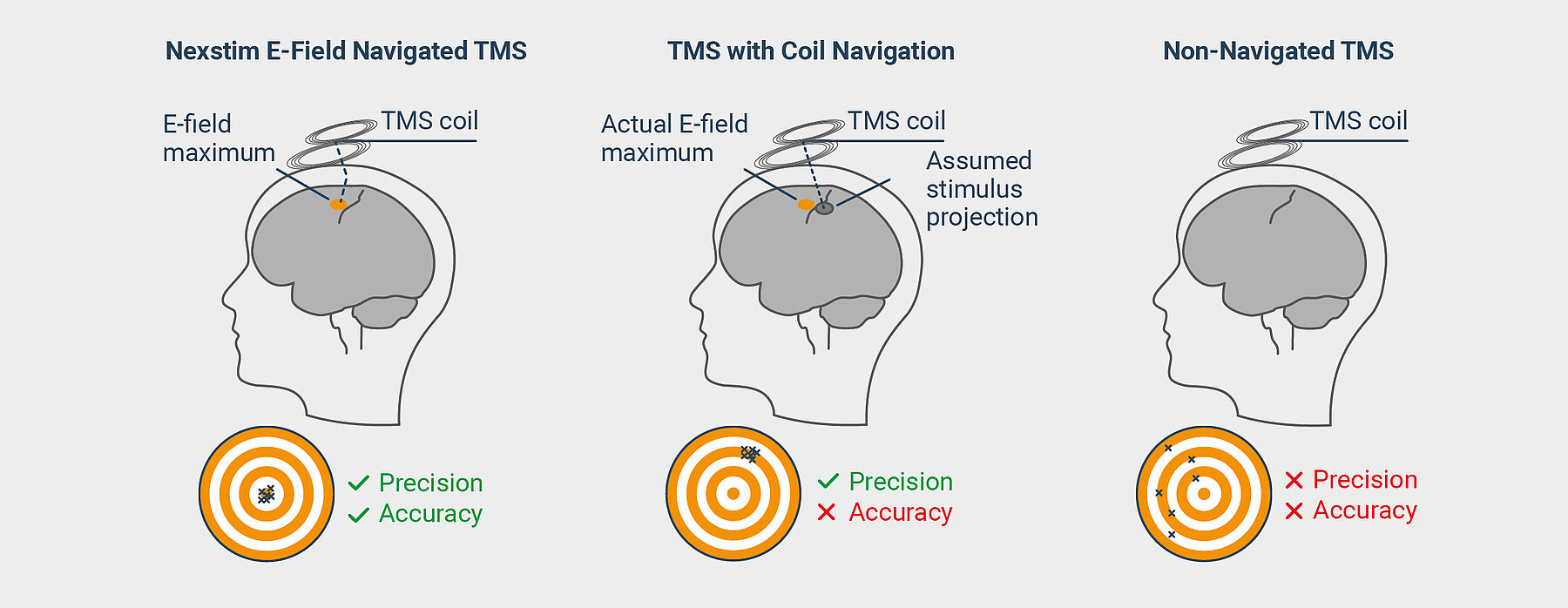
Coil navigation assumes that the stimulation projects perpendicularly from the coil. It does not account for the refraction of the stimulating electromagnetic field caused by bone and brain matter. Nexstim’s E-field navigation takes that refraction into account. Only through visualization of the stimulating E-field it is possible to achieve the precision and accuracy necessary to confirm the right dose is being delivered to the right location.
Interested in a live demo of nTMS?
Our team of physicians, researchers, and engineers is prepared to answer your questions. If you would like to learn more or set up a virtual demonstration for your team, please contact us!

SmartFocus® nTMS — a new non-invasive, non-drug therapy for depression.
Where to Stimulate?
In depression treatment, TMS is often targeted to the left dorsolateral prefrontal cortex (DLPFC). PET imaging studies in patients diagnosed with major depressive disorder (MDD) have shown lower metabolism, compared to controls, in their left DLPFC regions, which supports the concept of using TMS as a therapy to increase the excitability of the left DLPFC.1
Nexstim SmartFocus® nTMS navigation enables accurate targeting of the DLPFC. Without neuronavigation, research shows that the correct area is only targeted in approximately 30% of patients.2 Studies show that without navigation, treatments are unintentionally being delivered on average 2 cm posterior to the DLPFC 3.
Nexstim NBS 6 with SmartFocus® nTMS uses advanced algorithms based on mathematical modeling, taking into account both the shape and the composition of each patient’s individual brain. SmartFocus® nTMS displays the location and orientation of the maximum induced E-field in a 3D rendering, built from the patient’s own MRI head scan. The multi-sphere model has been scientifically validated to accurately determine the location and orientation of the maximum induced e-field in the brain and hence to target the stimulation to the intended spot with accuracy in the millimetre range. Without knowing the location of the maximum stimulating E-field in the brain, there is a risk of missing the target 70% of the time 2.
1. Bench, C. J. et al. The anatomy of melancholia: focal abnormalities of cerebral blood flow in major depression. Psychol. Med. 22, 607–615 (1992).
2. Herwig, U. et al. The navigation of transcranial magnetic stimulation. Psychiatry Res. 108, 123–131 (2001).
3. Ahdab, R. et al. Comparison of “standard” and “navigated” procedures of TMS coil positioning over motor, premotor and prefrontal targets in patients with chronic pain and depression. Clin. Neurophysiol. 40, 27–36 (2010).
How to Stimulate?
There are several factors affecting stimulation:
- patient’s head shape and size
- location of the coil
- orientation of the coil
- tilting angle of the coil
- distance between target and the coil
- coil rotation
Even if you are on target, if your TMS system is not calculating all these factors listed above, there is a very good chance that you are not, in fact, achieving optimal neuronal modulation. Nexstim SmartFocus® nTMS takes these factors into account. In other words, it calculates the E-field location and orientation. As the operator moves, turns or tilts the coil—even slightly—the system shows the updated location, orientation, and E-field data in real time and guides the operator to find the right positioning for the coil.
Personalizing the stimulation level
The effects of TMS are always dependent on the brain’s sensitivity level to stimulation. This state is unique for each patient but can easily be measured with Nexstim’s SmartFocus® nTMS technology by rapidly defining the patient’s resting motor threshold (MT) via the system’s integrated EMG. This gives you confidence that you are treating the intended location with a personalized dose to minimize any risk associated with overstimulation.
Yes, I would like to know more
Promising Initial Results: 76.2% Response Rate and 49.6% Remission Rate
Nexstim is reporting very promising clinical outcomes on depression patients treated with Nexstim nTMS. Of the first 403 patients completing treatment at clinical sites in the USA, 49.6% had achieved clinical remission and 76.2% had obtained a clinical response at end of treatment.
Clinical efficacy
10 Hz rTMS Stimulation protocol
The clinical efficacy and safety of 10 Hz rTMS stimulation of the left dorsolateral prefrontal cortex in the treatment of MDD has been studied in a sham-controlled multicenter clinical trial4 with an overall population of 301 patients meeting DSM-IV criteria in the diagnosis of MDD. MDD episode as defined by DSM-IV involves the nearly daily presence during the same two-week period of five or more of the following symptoms:
- Depressed mood most of the day as indicated by either subjective report or observation made by others
- Markedly diminished interest or pleasure in all, or almost all, activities most of the day
- Significant weight loss or weight gain, or decrease or increase in appetite
- Insomnia or hypersomnia
- Psychomotor agitation or retardation
- Fatigue or loss of energy
- Feelings of worthlessness or excessive or inappropriate guilt
- Diminished ability to think or concentrate, or indecisiveness
- Recurrent thoughts of death, recurrent suicidal ideation without a specific plan, or a suicide attempt or a specific plan for committing suicide.
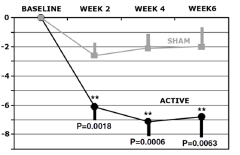
Efficacy of the treatment was established in retrospective analysis5 for the outpatient group (164 patients, aged 18-70 years) who had one adequate antidepressant treatment in the current episode but failed to achieve satisfactory improvement and were moderately to severely symptomatic. Efficacy of treatment, sham or active stimulation, was measured in total change in MADRS score at 2, 4 and 6 weeks into treatment when compared to baseline level (see the figure).
4. O’Reardon, J. P. et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol. Psychiatry 62, 1208–1216 (2007).
5. Lisanby, S. H. et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite randomized controlled clinical trial. Neuropsychopharmacology 34, 522–534 (2009).
Theta Burst Stimulation protocol
A multi-center clinical trial has demonstrated that the same clinical outcomes as with the 10Hz rTMS protocol can be obtained with rTMS provided in intermittent theta burst (iTBS) pattern6. The iTBS stimulation parameters used in this clinical trial and described below are the preprogrammed default iTBS stimulation parameters of the NBT system.
The safety and clinical efficacy of the 10Hz rTMS protocol (120% RMT stimulation intensity; 10 Hz frequency; 4s on and 26s off; 3000 pulses per session) has been compared to the safety and clinical efficacy of rTMS provided in iTBS pattern of triplet 50 Hz bursts repeated at 5 Hz; 2s on and 8s off; 600 pulses per session. In a multi-center non-inferiority trial of 414 patients with MDD, 205 patients were randomized to receive the 10Hz rTMS protocol and 209 the iTBS protocol, with both protocols delivered at the same site and intensity (120% RMT), differing only in stimulation pattern and total number of pulses. Treatment was provided 5 days per week for 4-6 weeks.
Both safety and efficacy data demonstrated iTBS to be non-inferior to 10 Hz rTMS for the treatment of depression.
The clinical remission and response rates at end of treatment were 32% and 49% in the iTBS and 27% and 47% in the 10Hz rTMS group (p=0.0005 for non-inferiority of iTBS). The 17-item Hamilton Rating Scale for Depression scores improved from 23·5 (SD 4·4) to 13·4 (7·8) in the 10 Hz rTMS group and from 23·6 (4·3) to 13·4 (7·9) in the iTBS group (adjusted difference 0·103, lower 95% CI −1·16; p=0·0011), which indicated non-inferiority of iTBS.
The most common treatment-related adverse event was headache in both groups (10 Hz rTMS: 131 [64%] of 204; iTBS: 136 [65%] of 208). There were no differences in the likelihood of adverse events between the 10Hz and iTBS group (Table 1, p>0.05 for each event type).
6. Blumberger, D. M. et al. Effectiveness of theta burst versus high‑frequency repetitive transcranial magnetic stimulation in patients with depression (THREE‑D): a randomised non‑inferiority trial. Lancet 391, 1683–1692 (2018).
Indications for use and patient safety
INDICATIONS FOR USE
CE mark and FDA clearance (K170902, K182700): Nexstim NBS 6 is indicated for the treatment of Major Depressive Disorder in adult patients who have failed to achieve satisfactory improvement from prior antidepressant medication in the current episode.
NBS 6 THERAPY SHOULD NOT BE GIVEN TO
- Patients with non-removable conductive, ferromagnetic, or other magnetic-sensitive metal anywhere in the head or within 30 cm (12 in) of the stimulation coil. Examples include cochlear implants, implanted electrodes or stimulators, aneurysm clips or coils, stents, bullet fragments, ocular implants, and stents.
- Patients who have an active or inactive implanted device (including device leads), including deep brain stimulators, cochlear implants, cardiac pacemakers, and vagus nerve stimulators. Contraindicated use could result in serious injury or death.
- Patients with increased intracranial pressure or patients with intracardiac lines, intravenous pumps, or dose calculators.
Failure to follow these restrictions could result in serious injury or death.
RISKS AND SIDE EFFECTS
Seizures (convulsions): Cortical magnetic stimulation runs the risk of inducing seizures; although they are rare. Under ordinary clinical use, the estimated risk of seizure is approximately 1 in 30 000 treatments (0.003%) or 1 in 1000 patients (0.1%).
Headache: The most common side effects reported during clinical trials are mild headache (~50% of TMS treatment group) and scalp pain or discomfort (35.8%). In general, headache and pain on the stimulation site have been generally mild to moderate and occurring less frequently after the first week of treatment. The reason for headache may be the tension of scalp and neck muscles due to an uncomfortable and stressful situation.
Muscle Twitching: You may feel twitches in the muscles of your arm, leg or face during the magnetic stimulation. This is a common sensation but not hazardous. The twitches will stop when the magnetic stimulation stops.
Skin Irritation: There is a small risk of mild skin irritation at the location where the muscle electrode sensors have been placed, but this usually consists of minor redness that will go away quickly after they are removed.
Changes in hearing: The loud “click” produced by the TMS stimulator can cause temporary hearing changes following treatment. This is prevented by wearing soft foam ear plugs during treatment. No problems with hearing due to TMS have ever occurred when earplugs have been properly worn
INEFFECTIVE TREATMENT
There is no evidence that single therapy sessions would improve mood. rTMS treatment effects in reducing depression are temporary, and patients may need to continue other forms of depression therapy. Relapse into depression is likely without follow-up treatment. Notify your doctor in case of worsening depression or suicidality.
CAUTION: SPECIAL POPULATIONS
All patients must be screened for the characteristics listed in this section and excluded without clear benefit or compelling clinical reason.
The safety and effectiveness of Nexstim TMS treatment has not been established in the following patient populations:
- Younger than 22 years or older than 70 years
- Suicide plan or recent suicide attempt
- History of concurrent use of electroconvulsive therapy (ECT) or vagus nerve stimulation (VNS)
- Depression secondary to a general medical condition or substance-induced
- Seasonal affective disorder
- History of substance abuse, obsessive compulsive disorder, or post-traumatic stress disorder
- A psychotic disorder, including schizoaffective disorder, bipolar disorder, or major depression with psychotic features
- History of increased intracranial pressure or head trauma
- Cardiac pacemakers, implantable cardioverter defibrillators, ocular implants, deep brain stimulators, vagus nerve stimulators, implanted medication pumps, intracardiac lines, or significant cardiac disease
- Pregnant or nursing
NBS 6 stimulation protocol for the treatment of major depressive disorder (MDD)
Pulse timing
10Hz
10 Hz bursts of 40 pulses (one burst is of 4 s duration)
One burst every 30 s, interval of 26 s
Total pulses in a sequence: 3,000
Total sequence duration: 18.8.-37.5 min
iTBS
50 Hz bursts of 3 pulses
5 burst per second, duration 2 seconds, 8 seconds interval
Total pulses in a sequence: 600
Total sequence duration: 3min 17s
Stimulation intensity
The intensity of the output of the stimulator should be set by operator to 120 % of the resting motor threshold (rMT) of the individual patient’s cortex. A patient’s rMT is determined in a two-step process.
First, single-pulse mapping of the primary motor cortex is used to find the coil location and orientation—the “hotspot”— giving the maximal EMG amplitude in the APB-muscle abductor pollicis brevis (APB) of the left thumb.
Second, targeting the hotspot, the operator uses Nexstim’s proprietary software-assisted stimulation sequence to determine the patient’s rMT. The NBS 6 software defines the rMT as the lowest level of stimulator output needed to elicit a >50 µV MEP response (peak-to-peak) in the muscle, as observed on EMG, 50% of the time from 10 stimuli.
It is important that the patient is fully relaxed during rMT finding and does not voluntarily activate (move) the hand or arm muscles.
Re-measurement of motor threshold
Re-measurement of rMT may be required if the treating physician suspects that the patient’s cortical excitability has changed. Since the patient’s individual hotspot is one of the patient’s vital parameters stored by the NBS 6, rMT re-measurement is a rapid procedure offering a reliable result for intra-patient comparison.
Frequently Asked Questions about TMS
Here are some answers to frequently asked questions we receive from physicians looking to open an nTMS clinic.
What is the scientific basis for rTMS as a therapy in depression?
rTMS is a locally acting, non-drug therapy directed to a specific neuronal region of the cortex, the left dorsolateral prefrontal cortex (DLPFC). Research has shown that the DLPFC is strongly interconnected with other brain circuits involved with mood and closely involved with the limbic system of the brain. The initial direct effects of rTMS are to excite the underlying neuronal circuits but it is believed that modulating the excitability of the DLPFC can have wide-ranging effects on brain activity.7,8,9
7. Bench, C. J. et al. The anatomy of melancholia: focal abnormalities of cerebral blood flow in major depression. Psychol. Med. 22, 607–615 (1992).
8. Fox, M. D. et al. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol. Psychiatry 72, 595–603 (2012).
9. Drysdale, A. T. et al. Resting‑state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med. 23, 28–38 (2017).
What are the benefits of rTMS in depression therapy?
Repetitive TMS (rTMS) therapy offers an attractive alternative treatment profile for patients with major depressive disorder (MDD) who have not responded to medication or those who find the side-effects of medication intolerable.
What are the risks and side-effects of rTMS?
rTMS treatment is generally well-tolerated with only minor side-effects following treatment (such as headache). rTMS has no known negative effects on cognition and there are normally no restrictions on driving a car to and from a treatment center, for example. After a treatment session, patients can return to their daily routine. No long-term side-effects from rTMS have been reported. Please see the complete list of possible risks and side effects either in the user manual or on Nexstim website.
How effective is rTMS in depression therapy? Which are the most important papers?
rTMS has been shown to be effective in treating MDD in patients who have not benefitted from anti-depressant medication. A large number of both independently conducted as well as company-sponsored studies have evaluated the efficacy of rTMS in the treatment of MDD. These studies have been summarized in multiple meta-analyses that have consistently supported the antidepressant efficacy of rTMS.
Studies in depression commonly use the Montgomery-Asberg Depression Rating Scale (MADRS) and the Hamilton Depression Rating Scale (HAMD) to measure outcomes.
In a well- conducted multisite study, the patient-reported remission rates were 26.5-28.7% and response rates were 41.5-56.4%.10
rTMS has been evaluated in a number of large multi-site, double-blinded sham-controlled trials. In these trials, patients have been received either daily rTMS to the left DLPFC or sham for between 3 and 6 weeks. A landmark trial was the NIMH (a US Government Agency)-funded study of 190 patients who had been diagnosed with MDD and a moderate level of resistance to medication. Using HAMD24 scoring, the trial showed that at week 6 active rTMS produced a clinically meaningful remission rate in depression that was approximately twofold higher than when the sham coil was used. 11
The first large trial of rTMS in Depression was a company-sponsored double-blind, multi-site study of 301 patients with MDD who had not benefited from prior treatment. The patients were randomized to receive either active or sham rTMS. Active rTMS was found to be significantly superior to sham rTMS on the MADRS at week 4 (with a post hoc correction for inequality in symptom severity between groups at baseline), as well as on the HAMD17 and HAMD24 scales at weeks 4 and 6. Response rates were significantly higher with active rTMS on all three scales at weeks 4 and 6. Remission rates were approximately two-fold higher with active rTMS at week 6 and significant on the MADRS and HAMD24 scales (but not the HAMD17 scale). There was a benefit shown for individuals who had previously failed just one depression medication regimen.12
10. Carpenter, L. L. et al. Transcranial magnetic stimulation for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. J. Clin. Psychiatry 73, 129–138 (2012).
11. George, M. S. et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham‑controlled randomized trial. Arch. Gen. Psychiatry 67, 507–516 (2010).
12. O’Reardon, J. P. et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol. Psychiatry 62, 1208–1216 (2007).
Is there any clinical evidence on the use of Nexstim nTMS in depression therapy?
Nexstim nTMS technology uses advanced algorithms based on mathematically modelling to allow you to not only see where you are stimulating but also calculate the stimulation intensity received by your target, giving you confidence that you are treating the intended location with a personalized dose, every time. The accuracy and precision that are based on this have been validated in neurosurgery.13 The exact same technology is used in depression therapy.
There are also initial, but promising results on the use of Nexstim nTMS technology in depression therapy. Clinical treatment outcomes of the 403 patients who have completed Nexstim navigated rTMS treatment with Nexstim NBT® system for major depressive disorder (MDD) were good: 49.6% were in remission at end of treatment and 76.2 % obtained a clinical response. (Read the report). While only a small sample, these outcomes are higher than what is usually reported for MDD patients treated with TMS: In a well- conducted multisite study, the patient-reported remission rates were 26.5-28.7% and response rates were 41.5-56.4%.10
13. Takahashi, S., Vajkoczy, P. & Picht, T. Navigated transcranial magnetic stimulation for mapping the motor cortex in patients with rolandic brain tumors. Neurosurg. 72, 131–138 (2013).
10. Carpenter, L. L. et al. Transcranial magnetic stimulation for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. J. Clin. Psychiatry 73, 129–138 (2012).
Why should I use navigated rTMS in depression therapy?
The use of a navigated TMS enables reliable, knowledge-based identification of an individual patient’s DLPFC. Once the DLPFC is identified, the system can automatically identify the optimal target prior to any repeat session with a high degree of precision. Without neuronavigation, a rule-of-thumb, like the 5 cm rule needs to be employed. However, this results in identifying the DLPFC correctly in only approximately 30% of patients.14 Although the motor cortex location corresponding to the thumb muscle twitch offers starting point for estimation, there is an additional error from locating the motor cortex manually.
Systems using the scalp 5 cm-rule ignore individual cortical anatomy, have an intrinsic error in locating the motor cortex for the starting point and offer no verification or documentation for intra-session or session-session targeting or dosing.
14. Herwig, U. et al. The navigation of transcranial magnetic stimulation. Psychiatry Res. 108, 123–131 (2001).
Why is the DLPFC the right spot for treating depression?
Targeting the DLPFC in depression is supported by more than 30 years of research. Lower metabolism and perfusion hemodynamics in the DLPFC, in patients vs. healthy controls, has been shown in hundreds of published research and clinical studies.
See, e.g.:
Drevets, W. C. & Raichle, M. E. Neuroanatomical circuits in depression: implications for treatment mechanisms. Biol. Psychiatry 44, 1055–1066 (1998).
Bench, C. J. et al. The anatomy of melancholia: focal abnormalities of cerebral blood flow in major depression. Psychol. Med. 22, 607–615 (1992).
How do you know where to stimulate?
Research has convincingly shown that the DLPFC is a valid target for intervention in mood-related disorders like MDD.7 The location of the DLPFC cannot be reliably determined in the individual patient by textbook-derived average distance from a scalp landmark or another functional target in the cortex. Given that stimulation might have undesirable effects off-target, it is important that the therapy provider is able to locate the DLPFC in the individual patient.
Having the individual patient’s own MRI head scan is the only way to reliably locate a central target to stimulate. Once an MRI is available, a navigated rTMS system can use the data to consistently target the same area of the patient’s brain with the same intensity for every treatment session.
7. Bench, C. J. et al. The anatomy of melancholia: focal abnormalities of cerebral blood flow in major depression. Psychol. Med. 22, 607–615 (1992).
Can pregnant women have rTMS therapy?
Safety and efficacy has not been established for use of rTMS in pregnancy or nursing, precautions should be taken.
Are there any limitations in terms of age for rTMS therapy?
RTMS therapy is only indicated for use in adults. Safety and efficacy has not been established for concurrent use of medication in patients younger than 22, precautions should be taken.
Can patients treated with rTMS take medication at the same time?
Safety and efficacy have not been established for concurrent use of medication with rTMS, precautions should be taken. However, in routine clinical practice patients are often on medication.
What are the contraindications for rTMS?
rTMS devices are contraindicated for use in patients who have conductive, ferromagnetic or other magnetic-sensitive metals implanted in their head or within 30 cm of the treatment coil. Examples include cochlear implants, implanted electrodes/stimulators, aneurysm clips or coils, stents, bullet fragments or jewelry. rTMS devices are also contraindicated for use in patients who have active or inactive implants, including deep brain stimulators, cochlear implants, vagus nerve stimulators or implanted device leads. Contraindicated use could result in serious injury or death.
An rTMS system should not be used by operators who are pregnant, have any electrical implants, or metallic implants anywhere in the upper body.
How long do patients have to try medication before rTMS treatment can be considered?
The FDA guideline is that a candidate for rTMS therapy needs to have failed at least one antidepressant medication.
How long does the therapy take and how many sessions are needed?
The total duration of the stimulation sequence in a treatment session is ~3-37.5 min depending on the decided treatment protocol. Nexstim NBS 6 with SmartFocus® nTMS technology is FDA cleared and CE marked for 3 min theta burst 50 Hz and 19 – 37,5 min 10 Hz protocols
Some additional time (minutes) is also required to co-register the patient with their MRI dataset. Treatment is normally applied daily for 4-6 weeks. In order to benefit from navigated rTMS with accurate targeting and dose calculation and delivery, there is a one-time set-up procedure of around 20 min, prior to the actual rTMS therapy.