Start by choosing your device's primary focus - diagnostics or therapy - and then customize it to meet your needs with additional features and modules:
Diagnostics
NBS 6 for Pre-procedural Brain Mapping
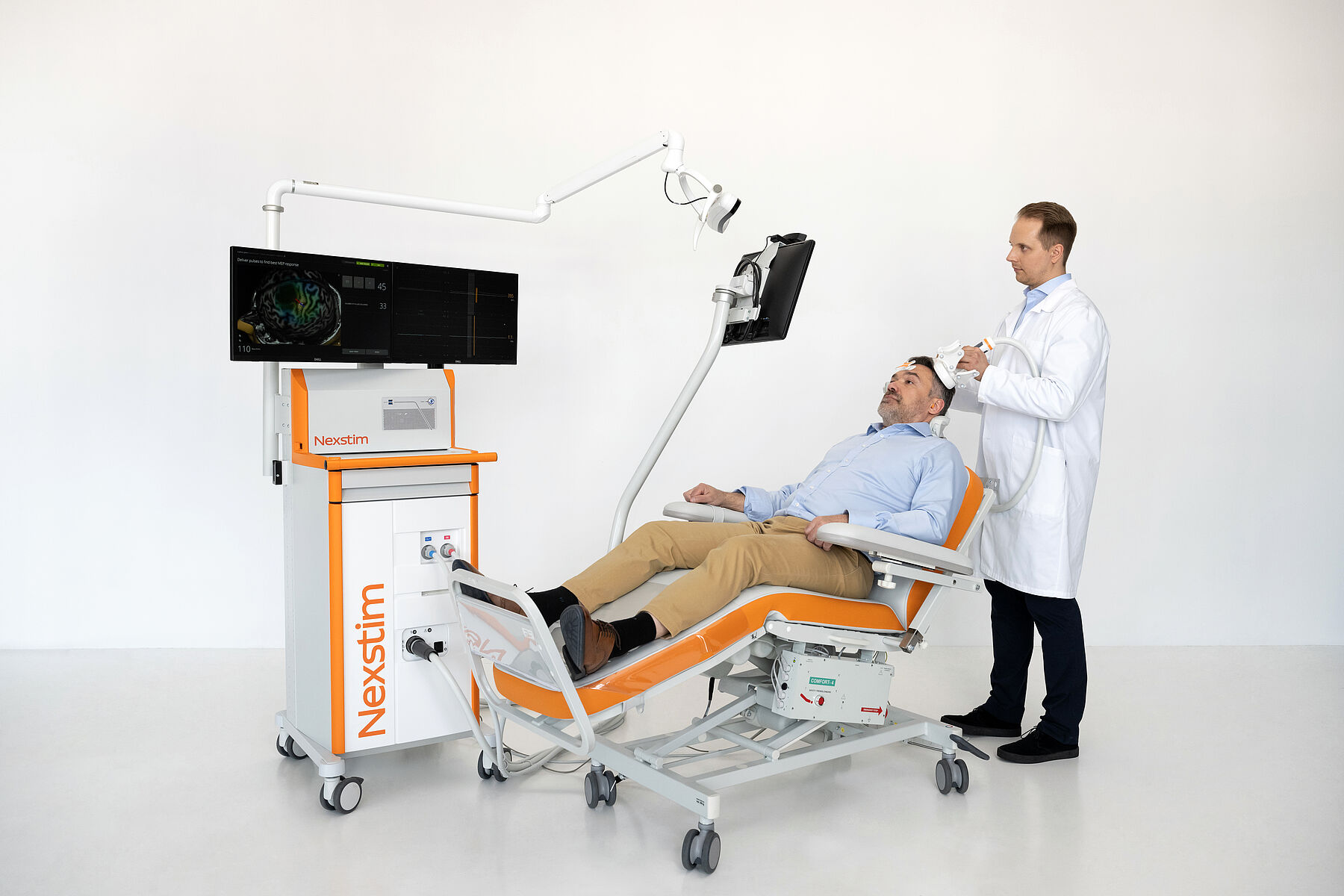
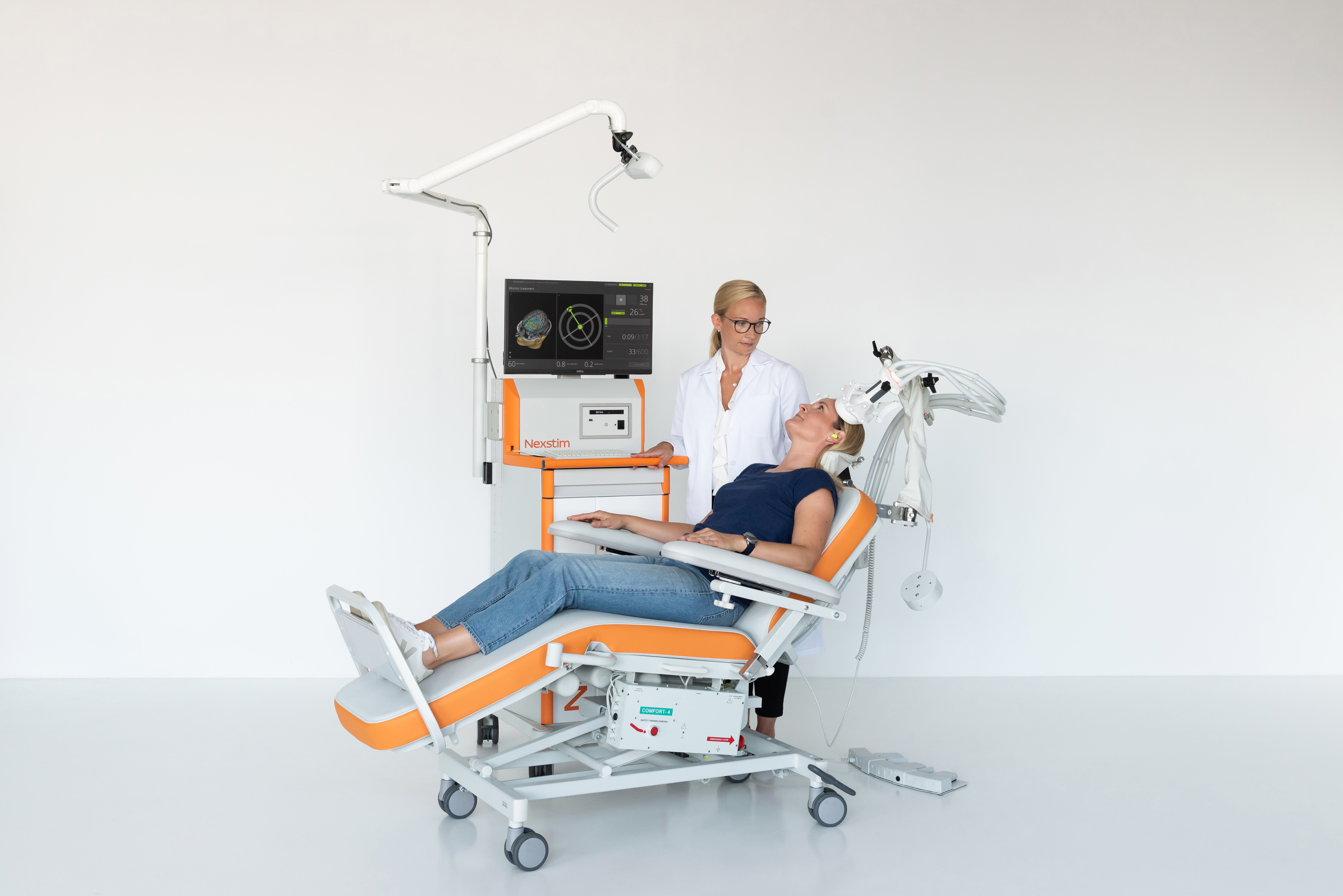
Includes an NBS 6 Base Unit: Motor Mapping, with:
- Nexstim NBS workstation, including:
- Arm-mounted tracking unit
- NBS computer and two screens
- Nexstim TMS stimulator and cooling unit
- EMG amplifier
- Foot pedal to control stimulator settings
- Digitizer pen
- Software workflow/application for motor mapping
- Adjustable patient chair
The system can be customized by adding a Therapy Module and/or a Speech Mapping Module as well as additional add-on features.
To receive more information on product configurations, please contact our team at info@nexstim.com, and we will help you select the right solution to meet the clinical and research needs of your team.
Diagnostics CE mark, FDA clearance, for information on other regional clearances contact Nexstim:
Intended purpose: NBS 6 is intended to be used for localization and assessment of the motor cortex and motor tract integrity for pre-procedural planning purposes. NBS 6 is intended to be used for localization and assessment of cortical areas of speech function for pre-procedural planning purposes.
Indications for use: NBS 6 is indicated for noninvasive mapping of the primary motor cortex of the brain to its cortical gyrus. NBS 6 provides information that may be used in the assessment of the primary motor cortex for pre-procedural planning. NBS 6 is indicated for noninvasive localizations of cortical areas that do not contain essential speech function. NBS 6 provides information that may be used in pre-surgical planning in patients undergoing brain surgery. Intraoperatively, the localization information provided by NBS 6 is intended to be verified by direct cortical stimulation. NBS 6 is not intended to be used during a surgical procedure. NBS 6 is intended to be used by trained clinical professionals.
See our NBS 6 product video for more details:
Therapy
NBS 6 for Depression Therapy
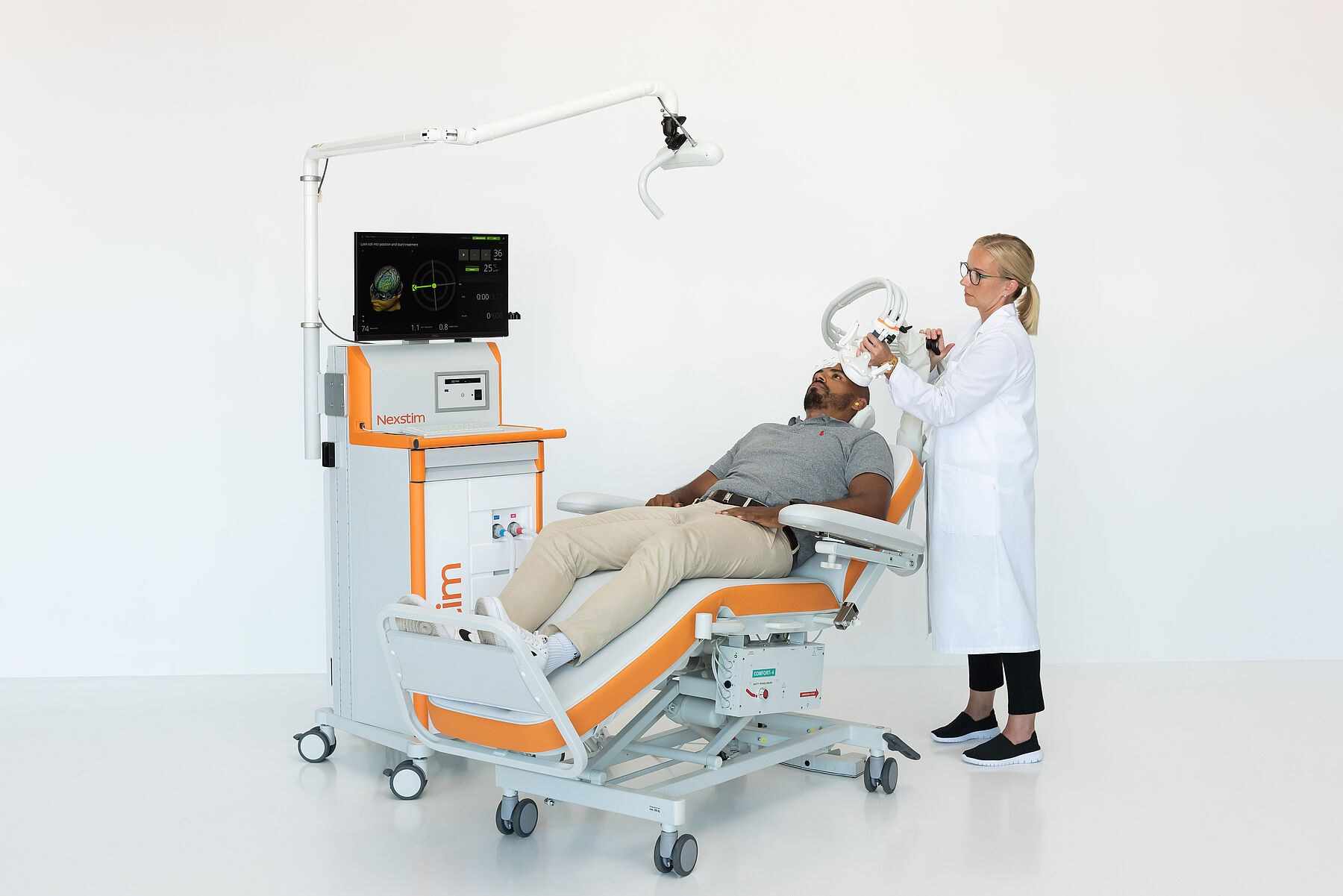
Includes an NBS 6 Base Unit: Therapy, with:
- Nexstim NBS workstation, including:
- Arm-mounted tracking unit
- NBS computer and touch screen
- Nexstim TMS stimulator and cooling unit
- EMG amplifier
- Foot pedal to control stimulator settings
- Digitizer pen
- Therapy software module, including convenient pre-programmed rTMS protocols and option to edit/create new protocols.
- Adjustable patient chair
- Coil positioning holder
The system can be customized by adding a Motor Mapping Module and a Speech Mapping Module as well as additional add-on features.
To receive more information on product configurations, please contact our team at info@nexstim.com, and we will help you select the right solution to meet the clinical and research needs of your team.
Major Depressive Disorder, CE mark, FDA clearance, for information on other regional clearances contact Nexstim:
Intended purpose: NBS 6 is intended to be used for treatment of major depressive disorder (MDD) by targeting and delivering noninvasive repetitive TMS stimulation to the patient's dorsolateral prefrontal cortex.
Indications for use: NBS 6 is indicated for the treatment of Major Depressive Disorder in adult patients who have failed to achieve satisfactory improvement from prior antidepressant medication in the current episode. NBS 6 is intended to be use by trained clinical professionals.
See our NBS 6 product video for more details:
Please read and accept terms before proceeding
This website and the information contained herein are not intended for, and must not be accessed by, or distributed or disseminated to, persons resident or physically present in Australia, South-Africa, Hong Kong, Japan, Canada or the United States of America, and this website does not constitute an offer to sell or the solicitation of an offer to buy or acquire, any shares, rights or other securities of the Company in Australia, South-Africa, Hong Kong, Japan, Canada or the United States or in any other country where it would be prohibited by local laws or other regulations.
The shares, rights or other securities of the Company referred to on this website have not been and will not be registered under the U.S. Securities Act of 1933, as amended, and may not be offered or sold in the United States absent registration or an applicable exemption from registration thereunder.
Access to the information and documents contained on the following websites may be illegal in certain jurisdictions, and only certain categories of persons may be authorized to access such information and documents. All persons residing outside of Australia, South-Africa, Hong Kong, Japan, Canada or the United States who wish to have access to the documents contained on this website should first ensure that they are not subject to local laws or regulations that prohibit or restrict their right to access this website, or require registration or approval for any acquisition of securities by them. The Company assumes no responsibility if there is a violation of applicable law and regulations by any person.
By clicking the link below in order to see the available documents you are considered to certify that:
- You are a resident and physically present outside Australia, South-Africa, Hong Kong, Japan, Canada or the United States;
- You are not a resident or physically present in any of the Member States of the European Economic Area (other than Finland or Sweden) having implemented the Directive 2003/71/EC of 4 November 2003 on the prospectus to be published when securities are offered to the public or admitted to trading and amending Directive 2001/34/EC (as amended) ("Prospectus Directive"), unless an exemption in the Prospectus Directive is applicable; and
- You are a resident and physically present (a) in Finland or Sweden or (b) outside Finland and Sweden and each of the jurisdictions referred to in clauses (1) through (2) above and, in that case, are authorized to access the information and documents on this website without being subject to any legal restriction and without any further action required by the Company.
By clicking the link below I confirm that I have read, understood and agree to comply with all restrictions set forth above:
Yes, I would like to know more
Frequently Asked Questions about TMS
Here are some answers to frequently asked questions we receive from physicians looking to open an nTMS clinic.