Benefits of nTMS brain mapping for epilepsy patients
- offers high spatial accuracy, similar to DCS1,2,3,7,8
- minimizes the need for 2-staged staged cases involving invasive ECS mapping2
- enables visualization of lesion-induced functional re-organization, including inter-hemispheric4
- multilingual mapping enables visualization of cortical regions associated with each language5
- helps optimize lead placement of subdural grids/strips4
- leads to better outcomes4,5
- uniquely suited for pediatric patients, who have limited brain mapping options available to them4
nTMS & MEG: A powerful combination in epilepsy surgery
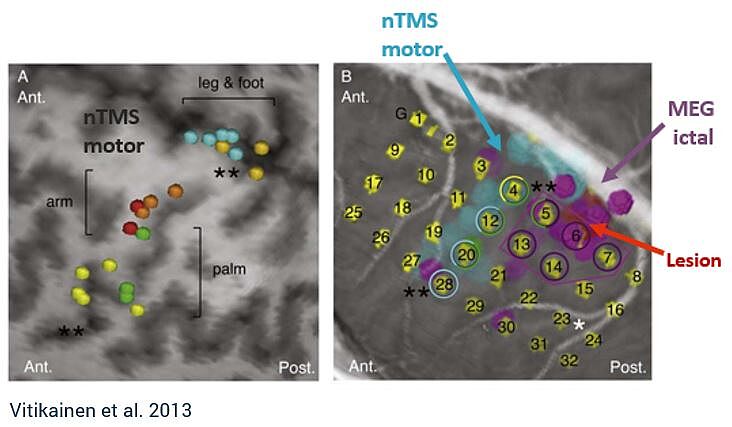
Combining MEG and nTMS can yield powerful results. MEG can be used to investigate the onset zone for epileptic seizures, whereas nTMS can provide the margin to functional tissue, providing safe resection zones.4 This combination may reduce the number of patients required to undergo two-staged cases for invasive ECS monitoring, which carries significant health risks.4,6
“[MEG and nTMS] can be added to the standard preoperative work-up and may even hold a potential to replace the ECS in a subgroup of patients with epilepsy who have the suspected epileptogenic zone near the sensorimotor cortex and seizures frequent enough for ictal MEG.” - Vitikainen et al.
How nTMS is used in practice
See how one major epilepsy program in the US is using Nexstim nTMS motor and language mapping preoperatively to advance epilepsy care.
Learn more about how nTMS is used by our community of experts:
Yes, I would like to know more
Considerations
Cortical magnetic stimulation runs the risk of inducing seizures - although they are rare. Single-pulse TMS, which is used in mapping the cortical representation areas of specific muscles, is generally considered a non-significant risk application. However, secondarily generalized or partial motor seizures have been induced in patients with stroke, intracranial brain lesions and major disorders of the central nervous system. The seizures have occurred either during the TMS stimulation or minutes after the end of the stimulation session.
In persons with epilepsy, lowered seizure threshold due to acute large brain infarctions, intracranial hemorrhage or trauma, or in persons on medication that lowers seizure threshold, Nexstim NBS System should only be used when clear benefit or compelling clinical reason exists. Visually monitor the patients for any signs of seizure or muscle twitching. If any signs appear, terminate the stimulation immediately and remove the stimulation coil from the patient. If necessary, adjust the patient chair to place the patient for seizure management.
Appropriate seizure management procedures should always be in place when using the Nexstim NBS System. Subjects should be fully informed of the nature of all risks and of what will happen to them should a seizure occur. Safety procedures for acute seizure management can include but are not limited to the following site requirements when Nexstim NBS System is used:
- presence of physician or nurse trained in seizure management
- presence of or ready access to, life-support equipment (oxygen, suction, blood pressure monitor, intravenous equipment, cardiopulmonary resuscitation (CPR) equipment), and
- access to anti-seizure medications.
References
1 Narayana et al., 2021. Clinical Utility of Transcranial Magnetic Stimulation (TMS) in the Presurgical Evaluation of Motor, Speech, and Language Functions in Young Children With Refractory Epilepsy or Brain Tumor: Preliminary Evidence, Front Neurol.
2 Raffa et al., 2019. The role of navigated transcranial magnetic stimulation for surgery of motor-eloquent brain tumors: a systematic review and meta-analysis. Clin Neurol Neurosurg.
3 Pasichnik et al., 2022. Discrepant expressive language lateralization in children and adolescents with epilepsy. Ann Clin Transl Neurol.
4 Vitikainen et al., 2009. Combined use of non-invasive techniques for improved functional localization for a selected group of epilepsy surgery candidates, NeuroImage.
5 Gibbs et al., 2021. Presurgical language mapping in bilingual children using transcranial magnetic stimulation: illustrative case, J Neurosurg Case Lessons.
6 Onal et al., 2003. Complications of invasive subdural grid monitoring in children with epilepsy, J Neurosurg.
7 Tarapore et al., 2013. Language mapping with navigated repetitive TMS: proof of technique and validation. Neuroimage.
8 Tarapore et al., 2013. Preoperative multimodal motor mapping: a comparison of magnetoencephalography imaging, navigated transcranial magnetic stimulation, and direct cortical stimulation. J Neurosurg.
Intended purpose & Indications for use
Pre-procedural mapping (CE mark, FDA clearance, for information on other regional clearances contact Nexstim):
Intended purpose: NBS 6 is intended to be used for localization and assessment of the motor cortex and motor tract integrity for pre-procedural planning purposes. NBS 6 is intended to be used for localization and assessment of cortical areas of speech function for pre-procedural planning purposes.
Indications for use: NBS 6 is indicated for noninvasive mapping of the primary motor cortex of the brain to its cortical gyrus. NBS 6 provides information that may be used in the assessment of the primary motor cortex for pre-procedural planning. NBS 6 is indicated for noninvasive localizations of cortical areas that do not contain essential speech function. NBS 6 provides information that may be used in pre-surgical planning in patients undergoing brain surgery. Intraoperatively, the localization information provided by NBS 6 is intended to be verified by direct cortical stimulation. NBS 6 is not intended to be used during a surgical procedure. NBS 6 is intended to be used by trained clinical professionals.
Major Depressive Disorder (CE mark, FDA clearance, for information on other regional clearances contact Nexstim):
Intended purpose: NBS 6 is intended to be used for treatment of major depressive disorder (MDD) by targeting and delivering noninvasive repetitive TMS stimulation to the patient's dorsolateral prefrontal cortex.
Indications for use: NBS 6 is indicated for the treatment of Major Depressive Disorder in adult patients who have failed to achieve satisfactory improvement from prior antidepressant medication in the current episode. NBS 6 is intended to be use by trained clinical professionals.
Chronic Neuropathic Pain (CE mark, for information on other regional clearances contact Nexstim):
Intended purpose: In adult patients suffering from chronic unilateral neuropathic pain, NBS 6 is intended to provide electric field navigated noninvasive repetitive TMS stimulation as therapy to alleviate pain. NBS 6 is intended to be used by trained clinical professionals.
Indications for use: NBS 6 is indicated for MRI-guided and electric field (or E-field) navigated, noninvasive repetitive TMS stimulation (rTMS) of the motor cortex as therapy to alleviate chronic unilateral neuropathic pain in adult patients. Nexstim NBS 6 is intended to be used by trained clinical professionals.
Nexstim NBS 6 is not cleared by the FDA for commercial use of the treatment of chronic pain in the United States, for investigational use only.

