Who We Are
Founded in Finland in 2000, Nexstim is a growth-oriented medical technology company operating in the international market. Nexstim has developed a world-leading non-invasive brain stimulation technology for navigated transcranial magnetic stimulation (nTMS) with highly sophisticated 3D navigation providing accurate and personalized targeting of the TMS to the specific area of the brain.
Nexstim has subsidiaries in the United States (Nexstim, Inc.) and in Germany (Nexstim Germany GmbH).
Over 230 NBS systems and over 90 systems that include therapy functionalities have been delivered to facilities worldwide for neurosurgical planning, multiple therapies, and research.
What We Do
Diagnostics
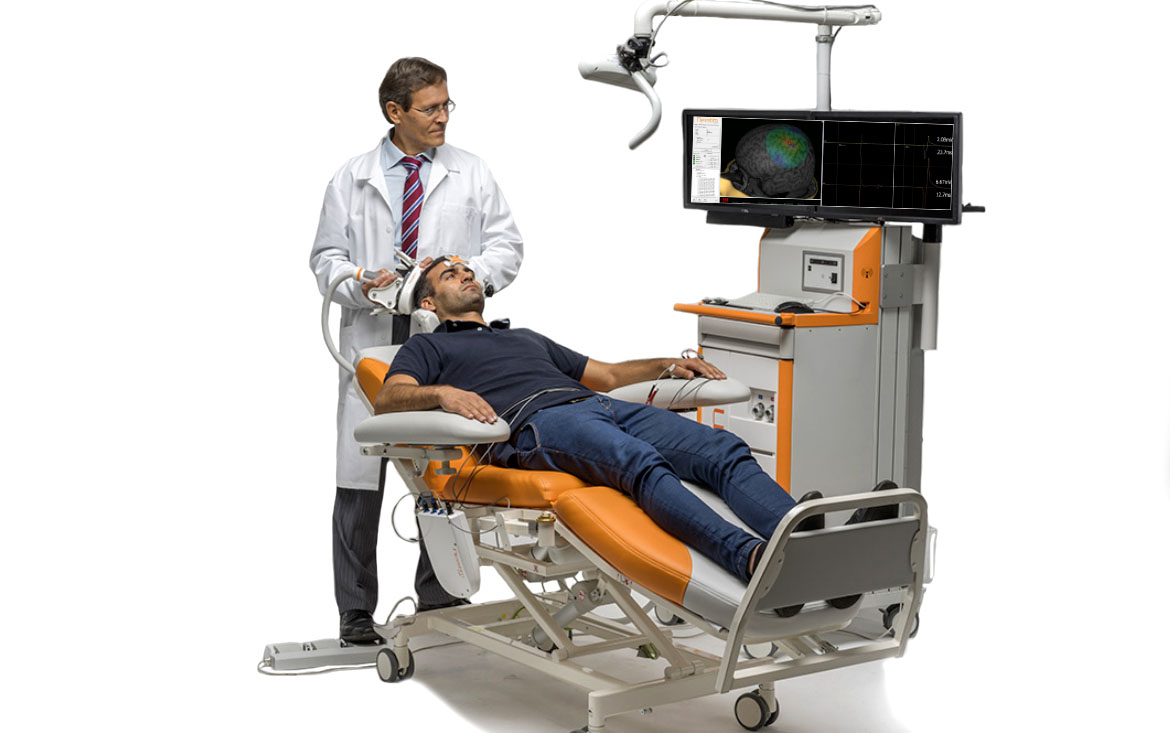
One of the most crucial pieces of information needed for neurosurgery is the tumor’s or other brain lesion’s location in relation to the essential functions and their connections in the patient’s brain. nTMS mapping with Nexstim’s system is used when the tumor is thought to be close to important motor and language areas in the patient’s brain. These brain maps are useful when deciding the treatment option.
Key authority approvals (NBS System 5):
- FDA approved for presurgical mapping of the speech and motor cortices of the brain.
- CE marked for presurgical mapping of the speech and motor cortices of the brain.
Therapy
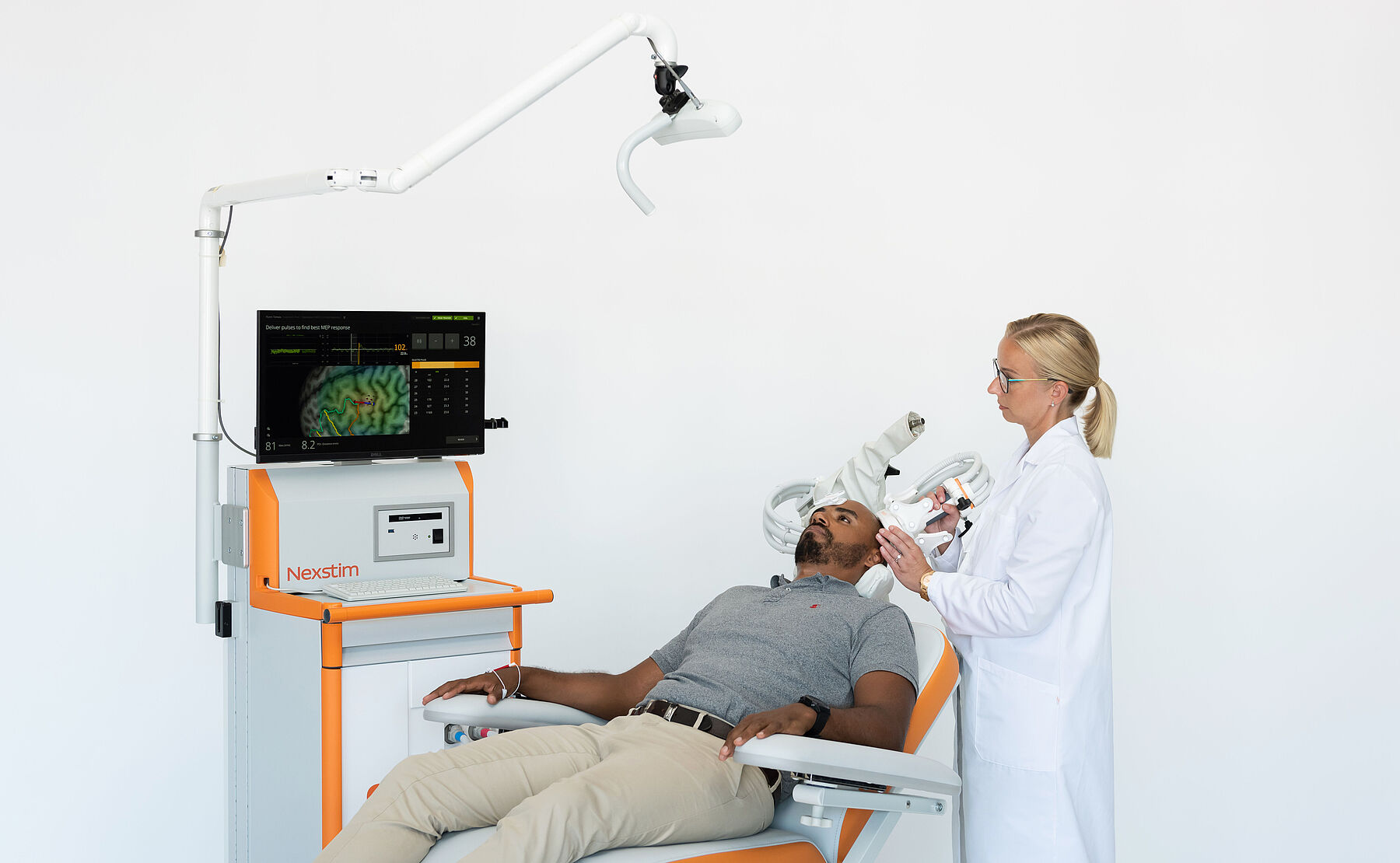
If pharmaceuticals are not working or a non-drug option is wanted, nTMS conducted with Nexstim’s system might be the answer for treatment of major depressive disorder or for chronic neuropathic pain.
Key authority approvals (NBT® System 2 and NBS System 6):
- FDA approved for the treatment of major depressive disorder
- CE marked for the treatment of major depressive disorder and chronic neuropathic pain.
Customers have the possibility of acquiring a system that has either diagnostic or therapy functionalities, or a system combination that enables the delivery of both applications in the same system.
Summary of CEO Mikko Karvinen's review of H1 2024:
"The first half of 2024 was in line with our expectations for Nexstim in terms of net sales growth and result improvement. In the first half of 2024 we achieved total net sale of EUR 3.2 (2.5) million with a 26.9% increase.
In line with our main strategic objective, we have continued to focus on profitable net sales growth. The sales forecast looks promising also for the full year 2024. Based on the strong first half and full-year business forecasts, we reiterate the guidance published in the annual report and estimate an improvement in the company's comparable net sales and operating result for the full year 2024.
Our second key strategic objective for 2024 is the launch of our new NBS 6 diagnostics system, enabling easy-to-use hardware and future add-on modules in the same system. The first launch in April-May 2023 concerned therapy applications. The first NBS 6 systems were successfully delivered to customers in the US in late 2023 and we have successfully continued delivering systems to the highly competitive US therapy market during 2024. We will continue the development of the system during the remainder of 2024, with the aim of launching diagnostic applications as part of the new NBS 6 system in line with our strategic objective.
Our third main strategic objective for 2024 is to expand the network of Nexstim partners and clinics primarily in the US and Europe.
With the expansion of the network of partner clinics, Nexstim systems would be installed in neuroscience clinics and possible investments would be made in clinic management service organizations in the vast US market. We are currently negotiating with new potential partner clinics in the US to grow our network of partner clinics in the important therapy market.
As evidence of the positive development of our strategic partnerships we announced in May that our US subsidiary Nexstim, Inc. has signed a strategic alliance agreement with Inomed Inc. to strengthen marketing, sales and application support collaboration between the two companies in the US. We believe that further significant growth can be achieved in the future particularly in the Diagnostics business through increasingly broad marketing and distribution partnerships. Through such strategic distribution partnerships, it is possible to cost-effectively reach an increasing number of customers with information about Nexstim's system and services, as well as potential representation in markets where it would not be as quickly and economically feasible to build on its own. The technological leadership of our products, their ease of use, and the scalability of additional modules create an attractive platform for growth through partnerships.
In line with our strategy to the end of 2024, Nexstim will continue to provide patients with personalized and effective treatment and diagnostics for severe brain diseases and disorders. We are updating our strategy and preparing to share more about this as the renewal work progresses. Based on the work already done so far, I believe that our strategy will continue to build on strong growth in both diagnostics and therapy. The growth and profitability performance of both our Diagnostics and Therapy businesses will play a key role in our efforts to minimize our future capital needs on our journey towards continued profitability."
Mikko Karvinen, CEO
Page last updated: 16 August 2024



